- Analysis Further Highlights the Potential for Zimura to Slow the Progression of Disease and Preserve Foveal Anatomy while Sparing the Foveal Center in Patients with GA -
IVERIC bio, Inc. (Nasdaq: ISEE) announced today a post-hoc analysis which evaluated various Geographic Atrophy (GA) growth parameters to explore the rate of disease progression within various regions in the fovea in a subset of patients from the GATHER1 Zimura® (avacincaptad pegol) Phase 3 clinical trial for the treatment of GA.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20220210005973/en/
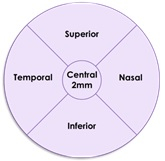
The accompanying schematic illustrates the five standardized regions (Graphic: Business Wire)
“Consistent with the overall results of GATHER1, in the new analysis a reduction in lesion growth in five standardized regions surrounding and including the central foveal area was observed for patients receiving Zimura 2 mg as compared to patients receiving sham over a period of 18 months,” stated Dhaval Desai, PharmD, Chief Development Officer of Iveric Bio. “We believe the observed pattern of reduction in GA growth is consistent with the natural history of the disease and recent clinical trial results in which complement inhibition has been observed to be associated with a greater reduction in GA growth in patients with non-foveal GA, which is known from the natural history to be faster progressing than foveal-involving GA. This analysis supports our expectation that we would see a greater reduction in growth away from the foveal center, reflecting the circumferential growth pattern typical for GA patients.”
“Geographic atrophy has a major impact on functional vision which could alter the quality of life and independence of affected individuals,” stated Pravin U. Dugel, MD, President of Iveric Bio. “We believe the results from this exploratory analysis are another step in studying the potential of Zimura to preserve central vision by slowing the progression of GA.”
The results of this subgroup analysis are consistent with the primary analysis results in the intent to treat population. The post-hoc analysis evaluated GA growth in five standardized regions in the retina for patients for whom images were available at relevant time points (n = 47 in the Zimura 2 mg group and n = 79 in the sham group).The five regions included the central foveal region, consisting of a 2 mm diameter circle around the foveal center point, and four quadrants, temporal, nasal, superior and inferior, in a concentric 8 mm diameter circle around the foveal center point. The accompanying schematic illustrates the five standardized regions.
The accompanying graphs illustrate the results of the analysis that will be presented today at the Angiogenesis, Exudation, and Degeneration 2022 meeting by Glenn J. Jaffe, MD, Director, Duke Reading Center Chief, Retina Division, Duke Eye Center, Robert Machemer Professor of Ophthalmology.
Iveric Bio will make the full set of slides of the presentation available on the Company’s website at https://investors.ivericbio.com/events-and-presentation at the start of the presentations at 11:00am Eastern.
About GATHER1 and GATHER2
The Company previously announced that GATHER1 showed Zimura (avacincaptad pegol) met its pre-specified primary efficacy endpoint with statistical significance in the Phase 3 clinical trial. The most frequently reported ocular adverse events reported with Zimura in this trial were related to the injection procedure. The Company expects topline data for GATHER2, a second Phase 3 clinical trial for Zimura for GA, to be available in the second half of 2022, approximately one year after the enrollment of the last patient in the trial plus the time needed for database lock and analysis. If 12-month results from GATHER2 are positive, the Company plans to file applications with the results from GATHER1 and GATHER2 to the U.S. Food and Drug Administration and the European Medicines Agency for marketing approval of Zimura for GA. There are no U.S. Food and Drug Administration or European Medicines Agency approved treatments available for patients with GA.
About Zimura
Zimura (avacincaptad pegol) is an investigational drug product and has not been approved for use anywhere globally. Zimura is designed to target and inhibit the cleavage of complement protein C5 and the formation of its downstream fragments, C5a and C5b. By inhibiting the formation of these fragments, Zimura is believed to decrease or slow the chronic inflammation and cell death associated with the retinal aging process by decreasing the formation of membrane attack complex (MAC) and inflammasome activity, thereby potentially avoiding or slowing the degeneration of retinal pigment epithelial cells. This potential mechanism is the rationale for Zimura as a potential therapy for geographic atrophy.
About Iveric Bio
Iveric Bio is a science-driven biopharmaceutical company focused on the discovery and development of novel treatments for retinal diseases with significant unmet medical needs. The Company is committed to having a positive impact on patients’ lives by delivering high-quality, safe and effective treatments designed to address debilitating retina diseases including earlier stages of age-related macular degeneration. For more information on the Company, please visit www.ivericbio.com.
Forward-looking Statements
Any statements in this press release or made during the presentation referenced herein about Iveric Bio’s future expectations, plans and prospects constitute forward-looking statements for purposes of the safe harbor provisions under the Private Securities Litigation Reform Act of 1995. Forward-looking statements include any statements about the Company’s strategy, future operations and future expectations and plans and prospects for the Company, and any other statements containing the words “anticipate,” “believe,” “estimate,” “expect,” “intend”, “goal,” “may”, “might,” “plan,” “predict,” “project,” “seek,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” and similar expressions. In this press release, the Company’s forward looking statements include statements about its expectations regarding its development and regulatory strategy for Zimura, its plans for additional indications, such as intermediate AMD, that the Company may pursue for the development of Zimura, the potential utility of Zimura and the clinical meaningfulness of clinical trial results. Such forward-looking statements involve substantial risks and uncertainties that could cause the Company’s development programs, future results, performance, or achievements to differ significantly from those expressed or implied by the forward-looking statements. Such risks and uncertainties include, among others, those related to the progress and success of research and development programs and clinical trials, and other factors discussed in the “Risk Factors” section contained in the quarterly and annual reports that the Company files with the Securities and Exchange Commission. Any forward-looking statements represent the Company’s views only as of the date of this press release. The Company anticipates that subsequent events and developments may cause its views to change. While the Company may elect to update these forward-looking statements at some point in the future, the Company specifically disclaims any obligation to do so except as required by law.
ISEE-G
View source version on businesswire.com: https://www.businesswire.com/news/home/20220210005973/en/
Contacts
Investor Contact:
Iveric Bio
Kathy Galante, 212-845-8231
Senior Vice President, Investor Relations
kathy.galante@ivericbio.com
or
Media Contact:
SmithSolve
Alex Van Rees, 973-442-1555 ext. 111
alex.vanrees@smithsolve.com













