- Starlight Therapeutics Inc., a Lantern subsidiary, will focus exclusively on the clinical development of therapies for CNS (central nervous system) and brain cancers with limited or no effective therapeutic options.
- Lantern's AI platform, RADR®, aided in the identification and accelerated development of Starlight's portfolio of therapeutic indications.
- The leading drug candidate, STAR-001, has demonstrated blood-brain barrier permeability, has favorable brain tumor bioavailability, and has shown nanomolar potency across an extensive number of in-vitro and in-vivo CNS and brain cancer models.
- Clinical trials in adult and pediatric CNS cancer indications are anticipated for late 2023 and early 2024.
- Globally, STAR-001’s targeted treatment indications represent an anticipated 500,000+ new cases each year and have a combined estimated annual market potential of over $6 billion (USD).
Lantern Pharma Inc. (NASDAQ: LTRN), a clinical-stage biopharmaceutical company using its proprietary RADR® artificial intelligence (AI) and machine learning (ML) platform to transform the cost, pace, and timeline of oncology drug discovery and development, today announced that it has formed a wholly-owned subsidiary, Starlight Therapeutics Inc. (“Starlight”), to develop drug candidate LP-184’s central nervous system (CNS) and brain cancer indications – including glioblastoma (GBM), brain metastases (brain mets.), and several rare pediatric CNS cancers. Starlight will refer to the molecule LP-184, as it is developed in CNS indications, as “STAR-001”. Combined, STAR-001’s targeted treatment indications are estimated to represent an annual global market potential of approximately $6.0 billion (USD) and over 500,000 global cases each year.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20230306005304/en/
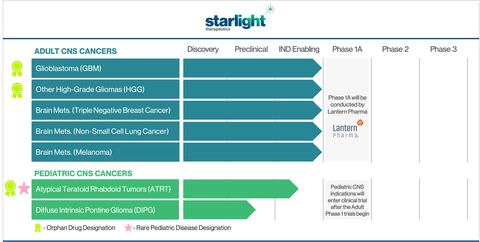
Starlight Therapeutics Pipeline of Adult and Pediatric CNS Cancer Indications (Graphic: Business Wire)
Starlight plans on establishing a leading CNS oncology franchise to develop the unique drug candidate STAR-001 for some of the most malignant and unaddressed primary and secondary CNS cancers. The programs being developed by Starlight were born from the analysis of billions of oncology-focused data points and by using Lantern’s AI platform, RADR®. STAR-001’s powerful anti-tumor mechanism of action, synthetic lethality, and collaborations with internationally recognized institutions, including the Kennedy Krieger Institute at Johns Hopkins and the Greehey Children’s Cancer Research Institute at UT Health – San Antonio, make it well positioned to advance in targeted and efficient clinical development programs. Starlight intends to pursue human clinical trials for multiple CNS indications starting in late 2023, building on prior IND-enabling studies and the Phase 1 clinical testing that will be conducted by Lantern.
“The formation of Starlight allows Lantern to put extreme focus on advancing STAR-001 through targeted clinical trials and dedicate increased time, resources, and personnel to progress one of the most promising drug candidates for CNS cancer patients in decades,” stated Panna Sharma, Lantern’s CEO and President. “Additionally, establishing Starlight as a wholly-owned subsidiary will increase the potential to partner with other biopharma companies who are looking to develop a franchise in CNS cancers and to further accelerate the progression of STAR-001 towards changing patient outcomes in this devastating set of diseases,” continued Sharma. “Since our initial discovery, in which we used large-scale, multi-omic network analysis from our RADR® AI platform, to validate that GBM was sensitive to LP-184 and that LP-184 had excellent blood-brain barrier permeability, our team has found, validated, and published on several additional pediatric and adult brain cancers that have shown early promise to LP-184, now STAR-001, in CNS cancers,” stated Sharma. “Our portfolio of opportunities and pipeline in CNS cancers has grown five-fold and includes multiple indications lacking any accepted standard-of-care. We believe that by focusing our efforts via Starlight Therapeutics we can accelerate and deepen our commitment to the CNS cancer patient community, while also creating the potential for meaningful additional upside for our investors,” concluded Sharma.
STAR-001 is a unique blood-brain barrier permeable small molecule that utilizes its powerful mechanism of action, synthetic lethality, to exploit common vulnerabilities in CNS cancers with DNA damage repair (DDR) deficiencies. The anti-tumor potential of STAR-001 has been demonstrated across an extensive number of in-vitro and in-vivo CNS cancer models, including GBM, brain mets., and atypical teratoid rhabdoid tumors (ATRT), and has been presented at leading conferences and publications including, the Society for Neuro-Oncology annual meeting, the American Association for Cancer Research annual meeting, and the Frontiers in Drug Discovery Journal. Highlights of STAR-001’s promising preclinical results from these presentations and publications are included below:
- Pharmacokinetic studies have shown STAR-001 to have 2X the bioavailability in brain tumors, compared to normal brain tissue, and to have 2X the bioavailability in brain tumors compared to the bioavailability of temozolomide (TMZ), the GBM standard-of-care (SOC) agent. Additional details from these experiments can be found here.
- In mice implanted with subcutaneous GBM cell-derived xenograft (CDX) tumors from models of the two major GBM subtypes, known as MGMT methylated and MGMT unmethylated, STAR-001 treatment resulted in 107% tumor growth inhibition in both tumor types. In these experiments, 75% of mice with tumors from the MGMT unmethylated GBM model and 30% of mice with tumors from the MGMT methylated GBM model were entirely tumor-free after STAR-001 treatment. Additional details from these experiments can be found here.
- STAR-001 has the potential for combination with the FDA-approved agent spironolactone to enhance STAR-001’s anti-tumor potency. In in-vitro GBM models with different MGMT methylation states, STAR-001 treatment with spironolactone significantly decreased STAR-001’s IC50 by 3-6X, resulting in IC50s in the low nanomolar range of 34-94nM. Additional details from these experiments can be found here.
- STAR-001 has been demonstrated to have nanomolar potency in brain mets. cell lines that originated from non-small cell lung cancer, melanoma, and breast cancer (HER2- and Triple Negative). Additional details from these experiments can be found here.
- STAR-001 treatment of mice implanted with ATRT CDX tumors, at either 2 mg/kg or 4 mg/kg doses (I.V.), showed near complete tumor growth inhibition between 82-91%, respectively. Additional details from these experiments can be found here.
Based on STAR-001’s demonstrated anti-tumor potential in CNS cancers, the FDA has granted STAR-001 Orphan Drug Designations (ODD) for malignant gliomas (including GBM) and ATRT. Additionally, STAR-001 was granted a Rare Pediatric Disease Designation for ATRT, which occurs in 60-70 pediatric patients a year in the US.
Starlight’s clinical development strategy will initially focus on progressing STAR-001 through early-stage clinical trials for adult recurrent high-grade gliomas (HGGs), including GBM. There have been no effective single-agent treatment options approved for GBM in nearly two decades. The current GBM standard-of-care agent, TMZ, is ineffective in MGMT unmethylated patients, who represent over 65% of all GBM patients. Starlight is planning several additional clinical programs for STAR-001 including adult and pediatric CNS cancers and combination regimens.
Commencing in mid-2023, Lantern is anticipating a Phase 1A basket trial for LP-184 (STAR-001), in a range of solid tumors including: recurrent brain cancers (including GBM and HGGs), metastatic CNS cancers (brain mets.), pancreatic cancer, and solid tumors with DDR deficiencies. The clinical development of STAR-001 in CNS cancers beyond the Phase 1A trial will be conducted exclusively by Starlight. Following the launch of Starlight, Lantern will continue to advance LP-184’s preclinical and clinical development for non-CNS indications (including pancreatic cancer and other solid tumors) and will also provide AI-driven bioinformatic and computational biology support to Starlight.
About Lantern Pharma:
Lantern Pharma (NASDAQ: LTRN) is a clinical-stage oncology-focused biopharmaceutical company leveraging its proprietary RADR® AI and machine learning platform to discover biomarker signatures that identify patients most likely to respond to its pipeline of genomically-targeted therapeutics. By targeting drugs to patients whose genomic profile identifies them as having the highest probability of benefiting from the drug, Lantern's approach represents the potential to deliver best-in-class outcomes.
Forward-looking Statements:
This press release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. These forward-looking statements include, among other things, statements relating to: the potential advantages of STAR-001; the potential advantages of our RADR® platform in identifying drug candidates and patient populations that are likely to respond to a drug candidate; our strategic plans to advance the clinical development of STAR-001; and expectations and estimates regarding clinical trial timing. Any statements that are not statements of historical fact (including, without limitation, statements that use words such as "anticipate," "believe," "contemplate," "could," "estimate," "expect," "intend," "seek," "may," "might," "plan," "potential," "predict," "project," "target," "model," "objective," "aim," "upcoming," "should," "will," "would," or the negative of these words or other similar expressions) should be considered forward-looking statements. There are a number of important factors that could cause our actual results to differ materially from those indicated by the forward-looking statements, such as (i) we have not commenced human clinical trials for any of the indications to be addressed by STAR-001, (ii) the risk that the results of our clinical trials for STAR-001 may not be successful or warrant further development, (iii) the risk that success in early phases of pre-clinical and clinicals trials does not ensure later clinical trials will be successful, (iv) our ability to fund the clinical trials and further development of STAR-001 and other product candidates and the availability of capital if and when needed, (v) the risk that none of our product candidates has received FDA marketing approval, and we may not be able to successfully initiate, conduct, or conclude clinical testing for or obtain marketing approval for our product candidates, (vi) the risk that no drug product based on our proprietary RADR® AI platform has received FDA marketing approval or otherwise been incorporated into a commercial product, and (vii) those other factors set forth in the Risk Factors section in our Annual Report on Form 10-K for the year ended December 31, 2021, filed with the Securities and Exchange Commission on March 10, 2022. You may access our Annual Report on Form 10-K for the year ended December 31, 2021 under the investor SEC filings tab of our website at www.lanternpharma.com or on the SEC's website at www.sec.gov. Given these risks and uncertainties, we can give no assurances that our forward-looking statements will prove to be accurate, or that any other results or events projected or contemplated by our forward-looking statements will in fact occur, and we caution investors not to place undue reliance on these statements. All forward-looking statements in this press release represent our judgment as of the date hereof, and, except as otherwise required by law, we disclaim any obligation to update any forward-looking statements to conform the statement to actual results or changes in our expectations.
View source version on businesswire.com: https://www.businesswire.com/news/home/20230306005304/en/
Contacts
Drew Sturtevant, Ph.D.
Sr. Associate, Scientific Affairs and Communications
contact@starlightthera.com
Please find more information at:
Lantern Pharma’s Website: www.lanternpharma.com
LinkedIn: https://www.linkedin.com/company/lanternpharma/
Lantern Pharma’s Monthly Newsletter – SPARK: Sign-up here
Twitter: @lanternpharma













