NEW YORK, April 25, 2024 (GLOBE NEWSWIRE) -- Tiziana Life Sciences, Ltd. (Nasdaq: TLSA) (“Tiziana” or the “Company”), a biotechnology company developing breakthrough immunomodulation therapies via novel routes of drug delivery, today announced for the first time, quantitative data showing improvement in White Matter Z-scores measured from PET images taken at 3 months in nasal foralumab treated patients with non-active secondary progressive multiple sclerosis (na-SPMS). White Matter Z-scores are a statistical measure used in neuroimaging studies to assess the integrity or abnormalities in structures of the brain.
Tarun Singhal, MBBS, M.D., Director of PET Imaging Program in Neurologic Diseases at Brigham and Women’s Hospital, a founding member of Mass General Brigham Healthcare System, and Associate Professor of Neurology at Harvard Medical School, stated, “Last week at the American Academy of Neurology annual meeting, we presented, for the first time, quantitative [F18]PBR06-PET data showing the dampening of microglial activation, an indicator of brain inflammation, in patients with non-active Secondary Progressive Multiple Sclerosis (na-SPMS) receiving intranasal foralumab. These data came from the open-label intermediate-sized patient population Expanded Access (ISPPEA) program. We calculated White Matter Z-scores to measure the effect of intranasal foralumab on microglial activation at baseline and then after foralumab treatment for three months. We saw reductions of 28% to 48%, indicating improvement in 5 out of 6 patients, and a 36% median reduction in White Matter Z-scores compared to baseline (see Figure 1). A peer-reviewed journal has published our recent work with newer [F18]PBR06-PET quantitation approaches.”[1]
The full publication in Clinical Nuclear Medicine can be found here: https://journals.lww.com/nuclearmed/fulltext/9900/glial_activity_load_on_pet_reveals_persistent.1077.aspx
“I am grateful for Dr. Singhal’s work advancing our intranasal foralumab program and the insight his work provides into the foralumab’s effect on brain inflammation. We are seeing this additional, encouraging evidence of intranasal foralumab’s effect after reporting that it attenuated microglial activation in na-SPMS patients with progression independent of relapse activity (PIRA) at 3 months as evaluated by [F-18]PBR06-PET and was associated with clinical symptom improvement or stability. Based on these positive results, a double-blind, placebo-controlled, dose-ranging study (NCT06292923) of intranasal foralumab in na-SPMS with [F-18]PBR06-PET as a primary endpoint with clinical measures of EDSS (disability) and MFIS (fatigue) is currently underway. I welcome the publication of Dr. Singhal’s [F18]PBR06-PET research paper, highlighting these combined findings in Clinical Nuclear Medicine,” commented Gabriele Cerrone, Chairman, acting CEO and founder of Tiziana Life Sciences.
Figure 1*
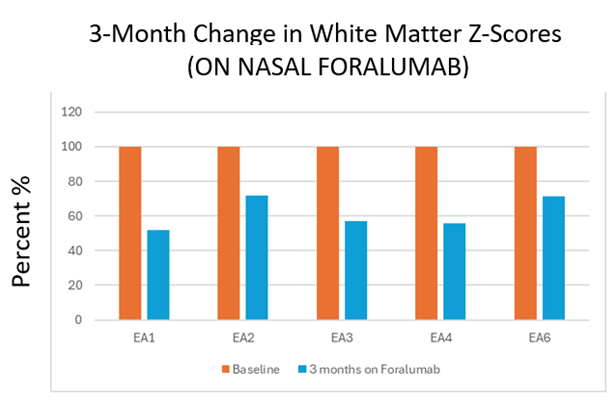
*EA5 showed a worsening in their White Matter Z-Score at three months during a pseudo-exacerbation of the patient’s trigeminal neuralgia.
About Foralumab
Activated T cells play an essential role in the inflammatory process. Foralumab, the only fully human anti-CD3 monoclonal antibody (mAb), binds to the T cell receptor and dampens inflammation by modulating T cell function, thereby suppressing effector features in multiple immune cell subsets. This effect has been demonstrated in patients with COVID and with multiple sclerosis, as well as in healthy normal subjects. The non-active SPMS intranasal foralumab Phase 2 trial began screening patients in November 2023. Immunomodulation by nasal anti-CD3 mAb represents a novel avenue for treating neuroinflammatory and neurodegenerative human diseases.[2],[3]
About Tiziana Life Sciences
Tiziana Life Sciences is a clinical-stage biopharmaceutical company developing breakthrough therapies using transformational drug delivery technologies to enable alternative routes of immunotherapy. Tiziana’s innovative nasal approach has the potential to provide an improvement in efficacy as well as safety and tolerability compared to intravenous (IV) delivery. Tiziana’s lead candidate, intranasal foralumab, the only fully human anti-CD3 mAb, has demonstrated a favorable safety profile and clinical response in patients in studies to date. Tiziana’s technology for alternative routes of immunotherapy has been patented with several applications pending and is expected to allow for broad pipeline applications.
For further inquiries:
Tiziana Life Sciences Ltd
Paul Spencer, Business Development and Investor Relations
+44 (0) 207 495 2379
email: info@tizianalifesciences.com
Investors:
Irina Koffler
LifeSci Advisors, LLC
646.970.4681
ikoffler@lifesciadvisors.com
[2] https://www.pnas.org/doi/10.1073/pnas.2220272120
[3] https://www.pnas.org/doi/10.1073/pnas.2309221120
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/4283dc55-3919-4ebb-a9dc-1348cfdea87c














